PERI-STRIPS

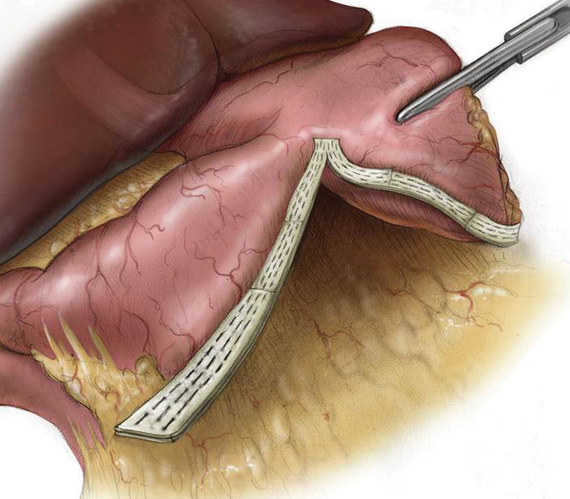
Confidence at the Staple Line
Over 20 years and 2.2 million implants later, PERI-STRIPS DRY with VERITAS is proven to improve clinical outcomes and reduce procedural complications.1,2,3
Strong From the Start (Burst Strength)*
An experimental study evaluating staple line seam integrity at intraluminal pressures in the immediate postoperative period with a canine model demonstrated:
Regardless of the operative techniques, the buttressed ileocolic anastomoses showed higher bursting pressures.*,8
Buttressed anastomoses never ruptured at the staple line.*,8
*Pre-clinical data results may not correlate to results in humans.
Additional Product Benefits
Outperforms Synthetic PGA Material
PERI-STRIPS DRY with VERITAS is derived from bovine pericardium and outperforms synthetic PGA material at the staple line over time in sleeve gastrectomy/gastric bypass patients.2
Reduced Risk of Staple Line Leaks and Bleeds
In a multicenter comparative use study, PERI-STRIPS DRY with VERITAS significantly lowered the risk of postoperative bleeding and leaks at the staple line compared to competitive products, over-sewing, and no staple line reinforcement.3
Fully Remodelable with Tissue Integration
Remodeling of PERI-STRIPS DRY with VERITAS entails two simultaneous processes that occur during normal wound healing: degradation of the VERITAS scaffold and deposition of new tissue and blood vessels, as demonstrated in pre-clinical studies*.4,5,6
*Pre-clinical data results may not correlate to results in humans.
Baxter takes safety of our products and patients very seriously. If you would like to report an adverse event with Baxter drugs (e.g. Tisseel, Artiss, …), you can contact Baxter directly: [email protected] or you can report it via authority’s website:
For Belgium: www.eenbijwerkingmelden.be or www.notifieruneffetindesirable.be
For Luxembourg: www.guichet.lu/pharmacovigilance
Any medical device product quality complaints (including medical device adverse incidents) relating to Baxter products should be sent to EMEA SHS Complaints Intake [email protected]